Ligands: In coordination chemistry and Complxometric Aanalysis
The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligand."
Metals and
metalloids are bound to ligands in virtually all circumstances, although
gaseous "naked" metal ions can be generated in high vacuum. Ligands
in a complex dictate the reactivity of the central atom, including ligand
substitution rates, the reactivity of the ligands themselves, and redox. Ligand
selection is a critical consideration in many practical areas, including
bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental
chemistry.
Ligands are
classified in many ways, including: charge, size (bulk), the identity of the
coordinating atom(s), and the number of electrons donated to the metal
(denticity or hapticity). The size of a ligand is indicated by its cone angle.
In Titration And Spectroscopy
Complexometric
titration (sometimes chelatometry) is a form of volumetric analysis in which
the formation of a colored complex ion is used to indicate the end point of a
titration. Complexometric titrations are particularly useful for the
determination of a mixture of different metal ions in solution. For example the use of EDTA (polydentate ligand) in determination of CaO and MgO for hardness analysis or
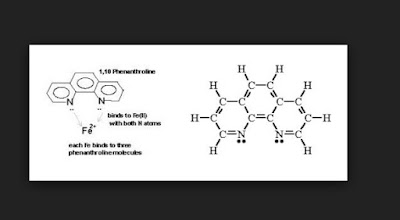
Spectroscopy example is the use of 1 10-phenanthroline (C12H8N2) to complex Iron III Oxide ion
Spectroscopy example is the use of 1 10-phenanthroline (C12H8N2) to complex Iron III Oxide ion









Post a Comment